Parathyroid hormone
| edit |
| Parathyroid hormone | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 PDB rendering based on 1bwx. |
|||||||||||||
|
|||||||||||||
| Identifiers | |||||||||||||
| Symbols | PTH; | ||||||||||||
| External IDs | OMIM: 168450 MGI: 97799 HomoloGene: 266 GeneCards: PTH Gene | ||||||||||||
|
|||||||||||||
| RNA expression pattern | |||||||||||||
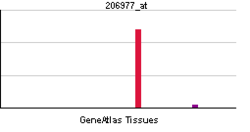 |
|||||||||||||
| More reference expression data | |||||||||||||
| Orthologs | |||||||||||||
| Species | Human | Mouse | |||||||||||
| Entrez | 5741 | 19226 | |||||||||||
| Ensembl | ENSG00000152266 | ENSMUSG00000059077 | |||||||||||
| UniProt | P01270 | n/a | |||||||||||
| RefSeq (mRNA) | NM_000315 | NM_020623 | |||||||||||
| RefSeq (protein) | NP_000306 | NP_065648 | |||||||||||
| Location (UCSC) | Chr 11: 13.47 - 13.47 Mb |
Chr 7: 113.18 - 113.18 Mb |
|||||||||||
| PubMed search | [1] | [2] | |||||||||||
Parathyroid hormone (PTH), parathormone or parathyrin, is secreted by the parathyroid glands as a polypeptide containing 84 amino acids. It acts to increase the concentration of calcium (Ca2+) in the blood, whereas calcitonin (a hormone produced by the parafollicular cells (C cells) of the thyroid gland) acts to decrease calcium concentration. PTH acts to increase the concentration of calcium in the blood by acting upon parathyroid hormone receptor in three parts of the body:[1] PTH half-life is approximately 4 minutes.[2] It has a molecular mass of 9.4 kDa.[3]
Contents |
Function
Regulation of serum calcium
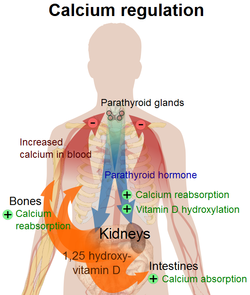
Parathyroid hormone regulates serum calcium levels through its effects on the following tissues:[4]
| Region | Effect |
| bone | It enhances the release of calcium from the large reservoir contained in the bones.[5] Bone resorption is the normal destruction of bone by osteoclasts, which are indirectly stimulated by PTH. Stimulation is indirect since osteoclasts do not have a receptor for PTH; rather, PTH binds to osteoblasts, the cells responsible for creating bone. Binding stimulates osteoblasts to increase their expression of RANKL, which can bind to osteoclast precursors containing RANK, a receptor for RANKL. The binding of RANKL to RANK stimulates these precursors to fuse, forming new osteoclasts which ultimately enhances bone resorption. |
| kidney | It enhances active reabsorption of calcium and magnesium from distal tubules and the thick ascending limb. As bone is degraded both calcium and phosphate are released. It also greatly increases the excretion of phosphate, with a net loss in plasma phosphate concentration. By increasing the calcium:phosphate ratio more calcium is therefore free in the circulation. [6] |
| intestine via kidney | It enhances the absorption of calcium in the intestine by increasing the production of activated vitamin D. Vitamin D activation occurs in the kidney. PTH up-regulates 25-hydroxyvitamin D3 1-alpha-hydroxylase, the enzyme responsible for 1-alpha hydroxylation of 25-hydroxy vitamin D, converting vitamin D to its active form (1,25-dihydroxy vitamin D). This activated form of vitamin D increases the absorption of calcium (as Ca2+ ions) by the intestine via calbindin. |
PTH was one of the first hormones to be shown to use the G-protein, adenylyl cyclase second messenger system.
Normal total plasma calcium level ranges from 8.5 to 10.2 mg/dL (2.12 mmol/L to 2.55 mmol/L).[8]
Regulation of serum phosphate
PTH reduces the reabsorption of phosphate from the proximal tubule of the kidney[6] which means more phosphate is excreted through the urine.
However, PTH enhances the uptake of phosphate from the intestine and bones into the blood. In the bone, slightly more calcium than phosphate is released from the breakdown of bone. In the intestines, which is mediated by an increase in activated vitamin D, the absorption of phosphate is not as dependent on vitamin D as is that of calcium. The end result is a small net drop in the serum concentration of phosphate.
Vitamin D synthesis
PTH increases the activity of 1-α-hydroxylase enzyme, which converts 25-hydroxycholecalciferol to 1,25-dihydroxycholecalciferol, the active form of vitamin D.
Regulation of PTH secretion
Secretion of parathyroid hormone is controlled chiefly by serum [Ca2+] through negative feedback. calcium-sensing receptors located on parathyroid cells are activated when [Ca2+] is low.[9] Calcium sensing receptors work by activating the phospholipase C pathway,[10][11] through a Gqα type of G protein, which ultimately increases intracellular concentration of calcium, which triggers vesicle fusion and exocytosis of parathyroid hormone. It also inhibits (not stimulates, as some[12] sources state) the cAMP-dependent pathway.[11]
Stimulators
- Decreased serum [Ca2+].
- Mild decreases in serum [Mg2+].
- An increase in serum phosphate (Since increased phosphate will complex with serum calcium to form calcium phosphate, which causes the Ca-sensitive receptors (CaSr) to think that serum Ca has decreased, as CaSR do not sense Calcium phosphate, thereby triggering an increase in PTH)
Inhibitors
- Increased serum [Ca2+].
- Severe decreases in serum [Mg2+], which also produces symptoms of hypoparathyroidism (such as hypocalcemia).[12]
Clinical significance
- A high level of PTH in the blood is known as hyperparathyroidism.
- If the cause is in the parathyroid gland it is called primary hyperparathyroidism. The causes are parathyroid adenoma, parathyroid hyperplasia and parathyroid cancer.
- If the cause is outside the gland, it is known as secondary hyperparathyroidism. This can occur in chronic renal failure. In secondary hyperparathyroidism, serum calcium levels are decreased, which causes the hypersecretion of PTH from the parathyroid glands. PTH acts on the proximal tubules in the kidney to decrease reabsorption of phosphate (increasing its excretion in urine, decreasing its serum concentration). NOTE: in chronic renal failure, the failing kidneys are unable to excrete phosphate in the urine. In this case of secondary hyperparathyroidism, serum calcium will be decreased, but serum phosphate will be increased.
- A low level of PTH in the blood is known as hypoparathyroidism. Causes include surgical misadventure (eg inadvertent removal during routine thyroid surgery), autoimmune disorder, and inborn errors of metabolism.
Measurement
PTH can be measured in the blood in several different forms: intact PTH; N-terminal PTH; mid-molecule PTH, and C-terminal PTH, and different tests are used in different clinical situations.
The average PTH level is 10-60 pg/ml.
See also
- Calcium metabolism
- Disorders of calcium metabolism
- Parathyroid hormone-related protein
References
- ↑ Physiology at MCG 5/5ch6/s5ch6_11
- ↑ Bieglmayer C, Prager G, Niederle B (October 2002). "Kinetic analyses of parathyroid hormone clearance as measured by three rapid immunoassays during parathyroidectomy". Clin. Chem. 48 (10): 1731–8. PMID 12324490. http://www.clinchem.org/cgi/content/abstract/48/10/1731.
- ↑ Prahalad AK, Hickey RJ, Huang J, et al. (June 2006). "Serum proteome profiles identifies parathyroid hormone physiologic response". Proteomics 6 (12): 3482–93. doi:10.1002/pmic.200500929. PMID 16705755.
- ↑ Coetzee M, Kruger MC (May 2004). "Osteoprotegerin-receptor activator of nuclear factor-kappaB ligand ratio: a new approach to osteoporosis treatment?". South. Med. J. 97 (5): 506–11. doi:10.1097/00007611-200405000-00018. PMID 15180028. http://meta.wkhealth.com/pt/pt-core/template-journal/lwwgateway/media/landingpage.htm?issn=0038-4348&volume=97&issue=5&spage=506.
- ↑ Poole K, Reeve J (2005). "Parathyroid hormone - a bone anabolic and catabolic agent". Curr Opin Pharmacol 5 (6): 612–7. doi:10.1016/j.coph.2005.07.004. PMID 16181808.
- ↑ 6.0 6.1 http://sprojects.mmi.mcgill.ca/nephrology/presentation/presentation5.htm
- ↑ Page 1094 (The Parathyroid Glands and Vitamin D) in: Walter F., PhD. Boron (2003). Medical Physiology: A Cellular And Molecular Approaoch. Elsevier/Saunders. pp. 1300. ISBN 1-4160-2328-3.
- ↑ Zieve, MD, MHA, David. "MedlinePlus Medical Encyclopedia: Serum calcium". National Library of Medicine, National Institutes of Health. http://www.nlm.nih.gov/medlineplus/ency/article/003477.htm. Retrieved 2009-02-01.
- ↑ Physiology at MCG 5/5ch6/s5ch6_9
- ↑ InterPro: IPR000068 GPCR, family 3, extracellular calcium-sensing receptor-related Retrieved on June 2, 2009
- ↑ 11.0 11.1 Coburn JW, Elangovan L, Goodman WG, Frazaõ JM (December 1999). "Calcium-sensing receptor and calcimimetic agents". Kidney Int. Suppl. 73: S52–8. PMID 10633465.
- ↑ 12.0 12.1 Costanzo, Linda S. (2007). BRS Physiology. Lippincott, Williams, & Wilkins. pp. 260. ISBN 978-0781773119. http://www.amazon.com/Physiology-Board-Review-Linda-Costanzo/dp/0781773113/.
Further reading
- Drüeke TB, Massy ZA (2003). "Advanced oxidation protein products, parathyroid hormone and vascular calcification in uremia". Blood Purif. 20 (5): 494–7. doi:10.1159/000065203. PMID 12207101.
- Parfitt AM (2003). "Parathyroid hormone and periosteal bone expansion". J. Bone Miner. Res. 17 (10): 1741–3. doi:10.1359/jbmr.2002.17.10.1741. PMID 12369776.
- Martin TJ (2004). "Does bone resorption inhibition affect the anabolic response to parathyroid hormone?". Trends Endocrinol. Metab. 15 (2): 49–50. doi:10.1016/j.tem.2004.01.002. PMID 15080150.
- Keutmann HT, Sauer MM, Hendy GN, et al. (1979). "Complete amino acid sequence of human parathyroid hormone". Biochemistry 17 (26): 5723–9. doi:10.1021/bi00619a019. PMID 728431.
- Keutmann HT, Niall HD, O'Riordan JL, Potts JT (1975). "A reinvestigation of the amino-terminal sequence of human parathyroid hormone". Biochemistry 14 (9): 1842–7. doi:10.1021/bi00680a006. PMID 1125201.
- Parkinson DB, Thakker RV (1993). "A donor splice site mutation in the parathyroid hormone gene is associated with autosomal recessive hypoparathyroidism". Nat. Genet. 1 (2): 149–52. doi:10.1038/ng0592-149. PMID 1302009.
- Handt O, Reis A, Schmidtke J (1993). "Ectopic transcription of the parathyroid hormone gene in lymphocytes, lymphoblastoid cells and tumour tissue". J. Endocrinol. 135 (2): 249–56. doi:10.1677/joe.0.1350249. PMID 1474331.
- Tonoki H, Narahara K, Matsumoto T, Niikawa N (1991). "Regional mapping of the parathyroid hormone gene (PTH) by cytogenetic and molecular studies". Cytogenet. Cell Genet. 56 (2): 103–4. doi:10.1159/000133059. PMID 1672845.
- Klaus W, Dieckmann T, Wray V, et al. (1991). "Investigation of the solution structure of the human parathyroid hormone fragment (1-34) by 1H NMR spectroscopy, distance geometry, and molecular dynamics calculations". Biochemistry 30 (28): 6936–42. doi:10.1021/bi00242a018. PMID 2069952.
- Arnold A, Horst SA, Gardella TJ, et al. (1990). "Mutation of the signal peptide-encoding region of the preproparathyroid hormone gene in familial isolated hypoparathyroidism". J. Clin. Invest. 86 (4): 1084–7. doi:10.1172/JCI114811. PMID 2212001.
- Nussbaum SR, Gaz RD, Arnold A (1990). "Hypercalcemia and ectopic secretion of parathyroid hormone by an ovarian carcinoma with rearrangement of the gene for parathyroid hormone". N. Engl. J. Med. 323 (19): 1324–8. PMID 2215618.
- Ahn TG, Antonarakis SE, Kronenberg HM, et al. (1986). "Familial isolated hypoparathyroidism: a molecular genetic analysis of 8 families with 23 affected persons". Medicine (Baltimore) 65 (2): 73–81. PMID 3005800.
- Tregear GW, van Rietschoten J, Greene E, et al. (1975). "Solid-phase synthesis of the biologically active N-terminal 1 - 34 peptide of human parathyroid hormone". Hoppe-Seyler's Z. Physiol. Chem. 355 (4): 415–21. PMID 4474131.
- Niall HD, Sauer RT, Jacobs JW, et al. (1974). "The amino-acid sequence of the amino-terminal 37 residues of human parathyroid hormone". Proc. Natl. Acad. Sci. U.S.A. 71 (2): 384–8. doi:10.1073/pnas.71.2.384. PMID 4521809.
- Andreatta RH, Hartmann A, Jöhl A, et al. (1973). "[Synthesis of sequence 1-34 of human parathyroid hormone]". Helv. Chim. Acta 56 (1): 470–3. doi:10.1002/hlca.19730560139. PMID 4721748.
- Jacobs JW, Kemper B, Niall HD, et al. (1974). "Structural analysis of human proparathyroid hormone by a new microsequencing approach". Nature 249 (453): 155–7. doi:10.1038/249155a0. PMID 4833516.
- Vasicek TJ, McDevitt BE, Freeman MW, et al. (1983). "Nucleotide sequence of the human parathyroid hormone gene". Proc. Natl. Acad. Sci. U.S.A. 80 (8): 2127–31. doi:10.1073/pnas.80.8.2127. PMID 6220408.
- Mayer H, Breyel E, Bostock C, Schmidtke J (1983). "Assignment of the human parathyroid hormone gene to chromosome 11". Hum. Genet. 64 (3): 283–5. doi:10.1007/BF00279412. PMID 6885073.
- Hendy GN, Kronenberg HM, Potts JT, Rich A (1982). "Nucleotide sequence of cloned cDNAs encoding human preproparathyroid hormone". Proc. Natl. Acad. Sci. U.S.A. 78 (12): 7365–9. doi:10.1073/pnas.78.12.7365. PMID 6950381.
- Hendy GN, Bennett HP, Gibbs BF, et al. (1995). "Proparathyroid hormone is preferentially cleaved to parathyroid hormone by the prohormone convertase furin. A mass spectrometric study". J. Biol. Chem. 270 (16): 9517–25. doi:10.1074/jbc.270.16.9517. PMID 7721880.
|
||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||